Abstract
The myelodysplastic syndrome (MDS) are a group of hematological malignancies with the propensity to develop into either acute myeloid leukemia (AML) or bone marrow failure (BMF). Dysregulation of immune and inflammatory responses has been implicated in MDS and other BMF disorders. There is significant evidence for IFNγ playing a key role in MDS and BMF syndromes. However, there are conflicting theories regarding the mechanism by which IFNγ promotes BMF. There is also very little information on the triggers that underlie upregulation of IFNγ in these BMF syndromes. Interstitial deletion of chromosome 5q is the most common cytogenetic abnormality observed in MDS, accounting for approximately 10% of all cases. Our lab has previously shown that miR-145, which is located on the minimally deleted region of chromosome 5q, targets Toll/Interleukin-1 receptor domain containing adaptor protein (TIRAP) - an innate immune adaptor protein. However, the role of TIRAP in marrow failure has not been well elucidated. In this study, we identify a novel role for TIRAP in dysregulating normal hematopoiesis through activation of Ifnγ.
Using bone marrow transplants in wild-type mice, we showed that constitutive expression of TIRAP in wild-type hematopoietic stem and progenitor cells (HSPC) caused peripheral blood cytopenia, suppressed the bone marrow endothelial niche and significantly reduced overall survival of the mice (median survival 9 weeks post-transplant) compared to controls (p < 0.0001). RNA-seq analysis of TIRAP expressing HSPC identified several proinflammatory cytokines to be significantly overrepresented. Geneset enrichment analysis (GSEA) identified the Ifnγ response as the single most significantly enriched pathway of the Hallmark genesets. To test the functional role of Ifnγ in TIRAP-mediated BMF, wild-type recipient mice were transplanted with TIRAP- HSPC from Ifnγ-/- donor mice. Mice that received TIRAP-transduced Ifnγ-/- HSPC were rescued from BMF, as evidenced by normalized blood cell counts and improved median survival (median survival 48.6 weeks) (Ifnγ-/- TIRAP vs. wild-type TIRAP: p = 0.0004). Interestingly, in our model of TIRAP induced BMF, myeloid rather than the conventional T and NK cells were the cells most responsible for the increased production of Ifnγ. Further, when we transplanted TIRAP expressing wild-type HSPC into NSG recipient mice, which are deficient in functional B, T and NK cells, the NSG mice developed BMF with pancytopenia in a similar time-frame as wild-type mice. This suggested that T and NK cells are not central for the development of TIRAP induced BMF.
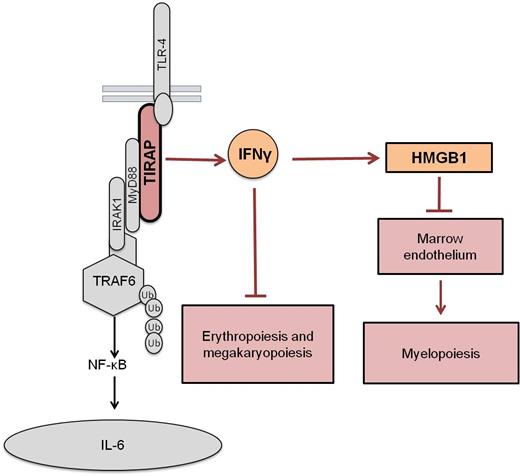
Delving deeper into the mechanism by which the TIRAP-Ifnγ axis causes BMF, we saw that while Ifnγ played a direct role in suppressing erythropoiesis and megakaryopoiesis, it played an indirect, Ifnγ receptor independent role on myelopoiesis. TIRAP-induced activation of Ifnγ released the alarmin, Hmgb1, which suppressed the marrow endothelial niche, which in turn promoted myeloid suppression. Overexpression of TIRAP in Ifnγ -/- background blocked Hmgb1 release. Further, blocking Hmgb1 in presence of TIRAP expression was sufficient to reverse the marrow endothelial defect and restore myelopoiesis in vivo.
Our findings highlight a novel, non-canonical effect of aberrant TIRAP expression via the Ifnγ-Hmgb1 axis on the endothelial cell component of the marrow microenvironment and hematopoiesis. Further understanding of this pathway would open up avenues for developing new therapies for BMF.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal